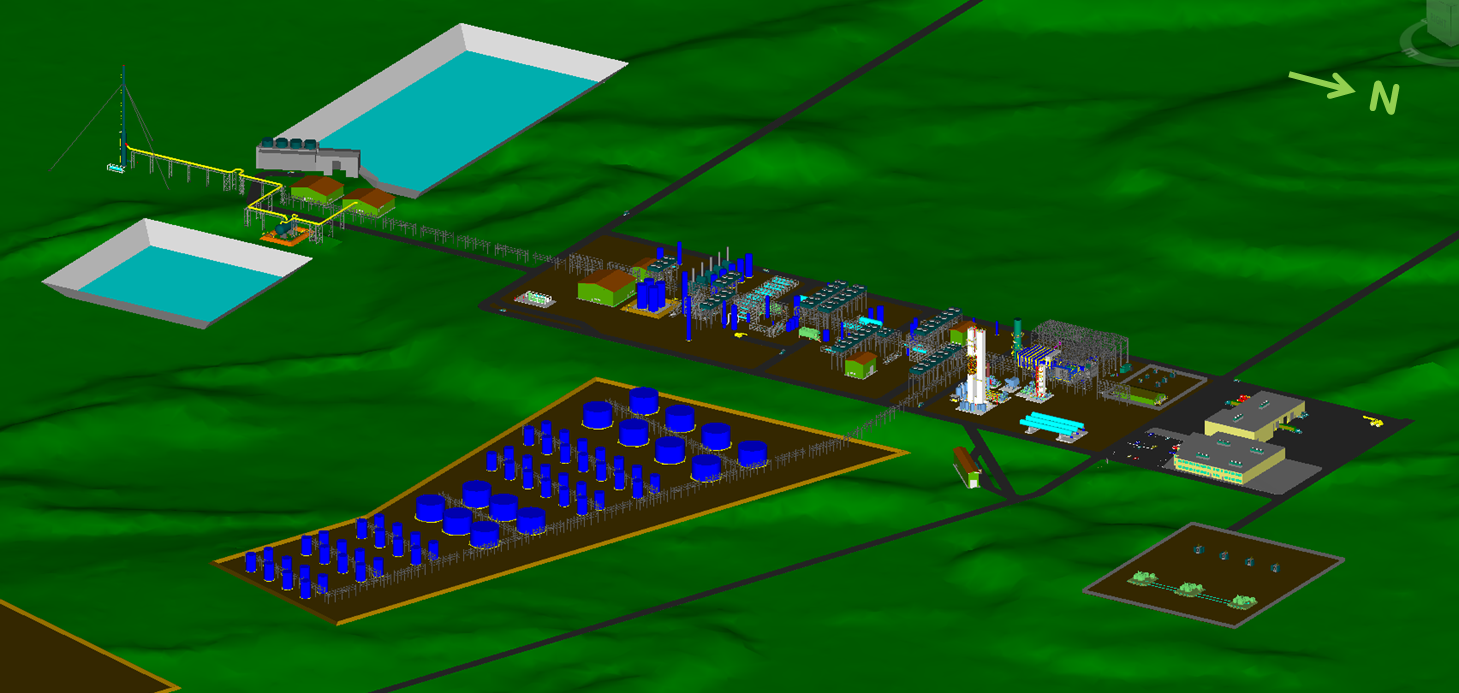
Gas to Clean Fuels Conversion Facility
Gas To Gasoline
To convert the natural gas to gasoline and other products, it must first be converted to methanol. The conversion of natural gas to methanol will be done using a process commonly used around the world.
Standard Methanol Plant:
The feedstock gas entering the methanol plant will undergo the following processes:
- Desulphurization: The methanol process is intolerant of sulphur in the feed gas, therefore the first step is to remove all traces of sulphur in an adsorbent bed. This step ensures that the final product (gasoline, LPG or DME) is completely sulphur free and therefore does not contribute to sulphur dioxide emissions that contribute to acid rain.
- Gas Reforming: Feedstock gas (pipeline quality sweet natural gas or manufactured ISCG synthetic gas) is reacted with steam and oxygen to form synthesis gas, a mixture of mainly hydrogen and carbon monoxide.
- Methanol Synthesis: The synthesis gas from the gas reformer is passed over a catalyst that results in the formation of methanol.
- Methanol Distillation: The produced methanol is then distilled, cooled and sent to temporary storage as a liquid prior to being sent to one of two other units for further processing.
Gasoline Conversion Unit
To produce gasoline, methanol will be sent to the Methanol to Gasoline Unit and undergo the following processes:
- Dimethyl Ether (“DME”) Synthesis: Methanol is passed over a catalyst to convert the methanol into DME and water. In the reaction, two molecules of methanol convert to one molecule of DME and one molecule of water.
- DME to Gasoline: The DME and water are then passed over a catalyst bed to convert the DME to gasoline and water. Each molecule of DME creates another molecule of water. Water created in these reactions is recovered and purified in order for it to be reused in the process, minimizing the fresh water demands.
Distillation:
The gasoline will then be distilled to remove LPGs, primarily propane and butane. Some light gas recovered is reused to generate power and steam for the process.
Gasoline Treating:
A final finishing step is used to meet particular specifications. This is a very small scale treating step when compared to the processing units in a typical oil refinery. The finished product is tank-ready gasoline that meets all the specifications outlined by the Canadian General Standards Board.
Dimethyl Ether (DME) Production
DME is gaining popularity worldwide as a diesel fuel substitute. It is produced from methanol in a simple process that is well established. To produce DME, methanol is sent to the DME Unit and undergoes the following steps:
- DME Synthesis: Similar to the first step in the gasoline synthesis process, methanol is heated and passed over a catalyst, whereby two methanol molecules combine to form one dimethyl ether (DME) molecule and one water molecule.
- DME Distillation: In the distillation process, unreacted methanol is removed from the fuel grade DME and returned to the methanol unit.
This is a long established, standard, simple process and is the basis for all worldwide DME manufacture today.
3D Model of 1st Phase of the Proposed
Gas to Clean Fuels Conversion Facility at Bickerdike